Education and training is an important pillar of a robust compliance program. To support that goal, OCEC developed this one-sheet guidance library in partnership with the research community to provide targeted and issue-specific guidance regarding key compliance obligations in order to assist researchers and administrators in our shared endeavor to promote the ethical and compliant conduct of research at USC. Each one-sheet provides an overview of the most important requirements to keep in mind, links to relevant university resources where additional information can be found, and key contacts to reach out to in the event you have additional questions.
In order to make the most effective use of the library, follow the steps below:
Select the stage in the research cycle you are interested in: The stages listed are Proposal Submission, Award and Account Establishment, After Research Commences, Closeout, Scientific Integrity, and Education Requirements. You can select more than one option.
Identify your position: Select the type of position from the “Select Your Role” dropdown menu to identify relevant guidance. You can select more than one option.
Select your one-sheet: You can either see a preview of the one-sheet or download it to your computer by selecting the “Click to Download” option.
You may also use the search box to look for one-sheets of interest.
If you have any questions, please do not hesitate to contact our office at compliance@usc.edu, and if you have suggestions for additional one-sheet topics, please submit your idea via the Research Compliance Suggestion Box.
| One-sheet Topic | Research Project – Stage | Recommended Audiences | Preview | Download | |
|---|---|---|---|---|---|
| 01 | Who May Be a Principal Investigator on Sponsored Research Projects at USC |
|
|  | Click to Download |
| 02 | Regulatory Research Committees |
|
|  | Click to Download |
| 04 | Cost Transfers |
|
|  | Click to Download |
| 05 | Cost Sharing |
|
|  | Click to Download |
| 54 | International Research Collaborations |
|
|  | Click to Download |
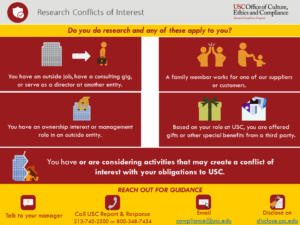
| 56 | Conflicts of Interest in Research - What to Disclose |
|
|  | Click to Download |
| 57 | Conflicts of Interest in Research - General Disclosure |
|
|  | Click to Download |
| 49 | Quick Guide on Federal Mandate to Disclose Foreign Research Relationships |
|
|  | Click to Download |
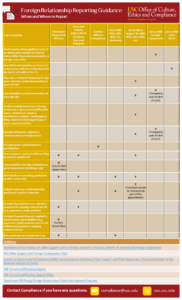
| 50 | Foreign Relationship Reporting Guidance: When and Where to Report |
|
|  | Click to Download |
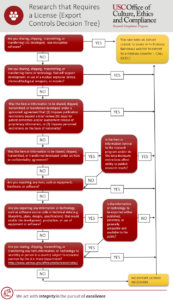
| 52 | Export Controls: Research that Requires a License – Decision Tree |
|
|  | Click to Download |
| 51 | NIH Disclosure Requirements Related to Foreign Research Activities |
|
|  | Click to Download |
| 14 | Recharge Centers and Specialized Service Facilities |
|
|  | Click to Download |
| 15 | Clinical Trials Registration Requirements |
|
|  | Click to Download |
| 16 | Closeout Reporting |
|
|  | Click to Download |
| 19 | Training: Grants Management |
|
|  | Click to Download |
| 24 | Travel and Fly America Act |
|
|  | Click to Download |
| 56 | Sponsored Research Travel Checklist |
|
|  | Click to Download |
| 32 | Sponsored Research Travel |
|
|  | Click to Download |
| 33 | No TikTok on Federal Contracts |
|
|  | Click to Download |
| 34 | NIH Data Management and Sharing: Required Elements of a Data Sharing Plan |
|
|  | Click to Download |
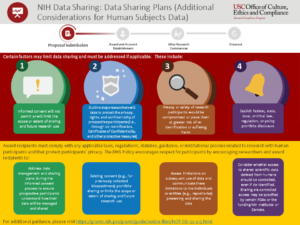
| 35 | NIH Data Management and Sharing: Data Sharing Plans (Additional Considerations for Human Subjects Data) |
|
|  | Click to Download |
| 36 | NIH Data Management and Sharing: Allowable Costs for Data Management and Sharing |
|
|  | Click to Download |
| 37 | NIH Data Management and Sharing: Selection of a Data Repository |
|
|  | Click to Download |
| 38 | NIH Data Management and Sharing: Selection of a Data Repository (Additional Considerations for Human Subjects Data) |
|
|  | Click to Download |
| 39 | NIH Data Management and Sharing: Method of Submission of Data Sharing Plans |
|
|  | Click to Download |
| 40 | NIH Data Management and Sharing: Benchmarking of Available Third-Party Repositories |
|
|  | Click to Download |
| 41 | Export Controls: International Travel and Export Controls |
|
|  | Click to Download |
| 42 | Export Controls: Technology Control Plans |
|
|  | Click to Download |
| 43 | Export Controls: Projects with Publication and/or Personnel Restrictions |
|
|  | Click to Download |
| 44 | Export Controls: Equipment Use and Export Controls |
|
|  | Click to Download |
| 45 | Export Controls: Research Activities in Iran |
|
|  | Click to Download |
| 46 | Export Controls: International Travel Safe Harbors |
|
|  | Click to Download |
| 47 | Export Controls: Foreign Visitors to Labs |
|
|  | Click to Download |
| 48 | Export Controls: Temporary Exports of Research Equipment |
|
|  | Click to Download |
| 53 | Clinical Trials Reporting Requirements |
|
|  | Click to Download |